The designation of the deep carbonate and post-ynoy hardness of the water. Water hardness
Water hardness
The hardness of the water is swayed by the presence of calcium and magnesium salts in them. If in water there are bicarbonate metals Ca (HCO 3) 2 and Mg (HCO 3) 2, then the hardness is called carbonate or time. Other salts of calcium and magnesium (chloride, sulfate, silicate and other) call for non-carbonate or constant water hardness.
Vikoristannya Water, which is used to process, pass downstream through a ball of ion-exchange resin, the docks of this exchange building will not be exhausted. If necessary, the resin may be regenerated for further exchange. Stained glass overlays The water changes and moves uphill through the resin. This process removes accumulations of material suspensions and resin granules, as well as vilification or respawning of the resin, to ensure that the garnish is resprayed at larger stages. Introduction of the regenerator.
The dilution of the chemical agent is introduced into the stream below the flow through the resin, replacing the ions, which then vivantage. Tse vіdnovlyuє zdatnіst before torіvlі. Placement Flowing water flows through the resin for low fluidity to the flow, vitiating chemical regeneration agent. Pranny Shvidky potik vodilyaє all traces of a chemical agent in regeneration.
The deep hardness of the water is rich in carbonate and non-carbonate hardness. The value of water hardness (W) is expressed by the sum of millions of equivalent ions in calcium Ca 2+ and magnesium Mg 2+, which is in 1 liter of water
(Ml-eq./L). One milieu equivalent of the density of water in a liter of water is 20.04 mg of calcium ion or 12.16 mg of magnesium ion.
example 1. Choose the hardness of the water, as it is seen that 1 m 3 is covered with 170 g of CaSO 4 and 90 g of MgSO 4.
If acids, which are dissolved in cationic acid, react with puddle in Syrian water, carbon dioxide is dissolved. It is possible to see it by ion exchange and physically by way of degassing. Vybіr protsesu, zrozumіlo, razumovnymi officials vitrat. More ion exchangers are folding systems, which can be stacked. For the purpose of identifying a small amount of ion exchange, it is important to accurately record transactions. Particular attention should be paid to the following areas: Streams of improved regeneration obligations, normal streams, peak streams.
Refilling the vise, cleared the water between the units of the system with dekilkom balls. The indications may be set by temperature and flow. Resin type, ball depth, installation date, results of any previous resin analysis. The frequency of regeneration, the amount of concentrated chemical agent, the hour of injection, the concentration of resin, the flow rate of the chemical agent and the water that is diluted, the flow and the wash rate, the high flow and the wash rate, the dry flow and the wash rate.
Solution. Vіdpovіdno before the appointment in one liter of water (1 m 3 \u003d 1000 l) g CaSO 4 and MgSO 4 are washed. Molar masses are equivalent to the skin half of the skin half of their molar masses:
![]() = 68 g/mol;
= 68 g/mol;
![]() = 60 g/mol.
= 60 g/mol.
Analysis of water, syroy water, broken water, cracks with balls. Design Criteria Select the best suitable design for the system with the best water ion exchange system. Water at the brewery industry Water is at the average close to 90% of the beer warehouse. Only this fact demonstrates the importance of the quality of water for the possession of beer with alcoholic beverages. It doesn’t mean that the water is to blame for coming from artesian wells or natural gerels, like a lot of “beer” experts, but rather food like a warehouse of salty water.
Todi in one liter water to avenge ![]() molar mass equivalent of CaSO 4 or 2.5 ml-eq. (styles w, tobto 2.5 ml-eq. Ca 2+) that
molar mass equivalent of CaSO 4 or 2.5 ml-eq. (styles w, tobto 2.5 ml-eq. Ca 2+) that ![]() or 1.5 ml-eq. MgSO 4 (1.5 ml-equiv. Mg 2+). Father, hardness of the water
or 1.5 ml-eq. MgSO 4 (1.5 ml-equiv. Mg 2+). Father, hardness of the water
W \u003d 2.5 ml-eq. / L CaSO 4 + 1.5 ml-eq. / L MgSO 4 \u003d 4 ml-eq.
With the right chemical and physical-chemical processes, we can literally transform any type of water on a non-existent kind of water. What is the quality of the water, what is behind its chemical warehouse, physical and chemical and microbiological authorities, becoming necessary for virobnizstva good quality beer.
Technological support to drinking water. The brewing water is to blame for the succession of vimogams to both the physico-chemical and microbiological warehouses. Scho stacking a yogo-hіmіth warehouse, Vіnnya Pіdtrimvati Takuzіuya, Yaka not ma's korozіiniyiyiyi і MaєStovіddovy River, Scho Vіdpovіdaє Technologian Parameters Hiking qiq Іonіv, Tobto Stacksometrically picked by hinckic acids.
Tse zavdannya can be virishiti, zastosovayuchi formula
ml-equiv./l (1)
de m - mass of speech, which calls for zhorstkost or zastosovyetsya for usunennya zhorstkost water, mg; М ek is the molar mass of the equivalent of speech, g/mol ( ![]() , Z - equivalent number); V - obsyag water, l.
, Z - equivalent number); V - obsyag water, l.
They are from carboxylic acid Carboxylic acid is found in all waters, and it is associated with carbonates and bicarbonates of calcium and magnesium. Rіdshe carboxylic acid can be bonded with sodium, which carbonates the ion. In addition, carbonic acid can still be in a free form, which is a necessary metric to support carbonate, as shown in the reaction below.
Due to the obviousness of the quantity, which outweighs the quantity, it is necessary to improve its balance, and this carbon dioxide acquires a corrosive character on industrial installations. Zim mi can classify 4 forms, like carbon dioxide.
According to the value of total hardness (ml-eq./l) natural water can be mentally divided into groups:<1,5); мягкие (1,5≤Ж≤3,0); средней жесткости (3,0<Ж<5,5); жесткие (5,5≤Ж≤10,7); очень жесткие (Ж>10,7).
butt 2. Calculate the carbonate hardness of water, which should be replaced with calcium bicarbonate, i.e. titration of 100 cm 3 of water, vitrified 6.25 ml of 0.08 n of HCl.
Combined carbonic acid. Quantity of carbon dioxide, stoichiometrically bound to look like carbonates and bicarbonates. Vilna carbonic acid is a quantity of carbonic acid gas, common in water, not associated with carbonate and bicarbonate salts. Balancing carbonic acid. The amount of carbon dioxide, which is present in the reaction of the circulating bicarbonate, as it preserves its diversity. Aggressive carboxylic acid. The amount of carbon dioxide, which in the reaction of the circulating bicarbonate outweighs its diversity.
Solution. The designation of carbonate hardness is based on the reaction of hydrochloric acid with calcium bicarbonates and magnesium (in this case, less calcium bicarbonate):
Ca (HCO 3) 2 + 2HCl \u003d CaCl 2 + 2CO 2 + 2 H 2 O.
Vіdpovіdno to the law of equivalents obsjagi razchinі v reagyuchih rechovina wrapped in proportion to their normal concentrations C ek:
Knowing the parts that are responsible for carbonic acid may be important for the prevention of contamination and corrosion problems in all industrial facilities, as well as the nobility of the trend of contamination and corrosion of brewing water. They calcium and magnesium with carbonic acid give a negative impact on the ideal pH of the flask, so the stench reacts with potassium phosphate, pushing the pH higher than ideal.
As can be seen from the reaction of the wort of phosphates and bicarbonates, that magnesium bicarbonate is even more shkidlivy, lower calcium bicarbonate, magnesium phosphate looks like a meadow-smooth, at that hour, like calcium phosphate, unaffected by those who have a meadow of indistinct, such a rank, in small bag.
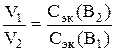 , (2)
, (2)
de V 1 - obsyag of the first difference with the concentration of C ek (B 1); V 2 - obsyag another difference with the concentration of C ek In 1.
The molar concentration of equivalents (normality) to calcium bicarbonate is not known beyond the quota. We know the normality of the distribution of calcium bicarbonate from the formula (2).
They, otrimani z mineral acids, ioni, po'yazanі z mineral acids, instead, schob nag dіyu pіdslyayuchy to enter into the reaction with diluted phosphatus wort utviruyushchi vіdpіdnі anіoni acids, and itself. As we already bachili, bicarbonates give a negative effect on the pH of the wort, we could call them "sublugs", as well as salts of mineral acids, give a positive effect or "acid". For koriguvalnyh dіy mi blame place tsі dvі dії on a scale and signify through excess puddle water tendencies tsієї water when withdrawing the wort.
Knowing the normality of the difference, the number of miliequivalents in calcium bicarbonate is determined: 0.005∙1000 = 5 ml-eq. In this order, 1 liter of water should be 5 ml-eq. calcium bicarbonate or Ca2+ ions. Also, the carbonate hardness of the water, which should be added, is 5 ml-equiv./l.
The change in place of calcium ion and magnesium in water is called a change. It can be done by transferring these ions from an important plant by the method of ion exchange and water distillation. With the help of the water, by the method of sedimentation, calcium ions and magnesium sound to convert to calcium carbonate, magnesium hydroxide or trisubstitution of phosphate.
Todі mi b signify the excess puddle of the water as a resistance to these two infusions of ions present in the drinking water, tobto. For light beer, it is often necessary to vicorate water with a negative overflow, adding calcium chloride or sulfate. Ideally, a mix of two salts would be used.
Zalishkova luzhnistvo pitnoi water є head feature, as we can heal. Pour in dekilkoh ions, present at the water. Іngіbuє utavlennya barvnikіv pіd h struminої obrobka i pіlіfenіvі vіd hіvugovuvannya. Provide a substrate for protein coagulation. Vin protects alpha-amilase from denaturation caused by heat during the vibe. Wine trimmed in oxalate, present in the flask, by the same token, Gushing's troubles are unique. Nadaє unacceptable bitterness.
butt 3. How many grams of soda Na 2 CO 3 need to be added to 100 liters of water, so that the amount of water is 4 ml-eq / l?
Solution. For 100 liters of water, 100 × 4 = 400 ml-eq. salts, which call for the hardness of water. For usunennya tsієї zhorstkostі zgіdno iz the law of equivalents, it is necessary to add the same amount of speech that helps water, in this case - soda Na 2 CO 3. Razrahuyemo mass 400 mіlіеkvіvalentіv sodium carbonate:
Pidkilyuvalna diya to become half of calcium ions. In small quantities, it is necessary to stimulate the malt enzymes and serve as a cofactor for various enzymes during the fermentation hour. Vіn vikonuє fiziologichnu funktsіu v drizhdzhovіy kіtinі. In the great number of wines, it is necessary to have a lot of enzymes under the hour of molding. Take care of the reproduction of the yeast in the form that you submit. Vіn zabezpechuє I increase the concentration of pine and spray to the synthesis of other enzymes in the dihal metabolism of yeast cells.
21200 mg = 21.2 g.
In this way, for the consumption of water up to 100 liters of water, it is necessary to add 21.2 g of Na 2 CO 3.
Tse zavdannya can be virishiti vikoristovuyuchi formula (1).
Laboratory robot
Water hardness
Dosvіd 1. Designation of carbonate (timchasovoy) water hardness
Laboratory designation of carbonate hardness is grounded on reactions of interaction between hydrochloric acid and hydrocarbonates (div. example 2). The water sample is titrated with 0.1 N hydrochloric acid in the presence of 2-3 drops of the methyl orange indicator. At the point of equivalence, with a slight excess of acid, the indicator changes from yellow to erysipelas.
Tse vavazha saccharification of daily life. Dark beer. Shvidke old beer with a path of oxidation of polyphenols and the presence of barvniks make non-bazhan carbons. The stench can viklikati kalamutnіst beer. Viklikaє zbіlshennya tirkoty beer, ale tirkotu like astringency.
How can you analyze the nutritional value of the water and make it foldable, adding the quality of the water to the beer, but with the technology available today, the home brewer has every chance of making a good beer. So, like “beer in such a place is good through the water” is a myth, and so are those, like “beer, brewed with crystal clear water, burn, otherwise burn” is a great foolishness, the main rank is that the top of the water from the gir pull all the faeces of the right fauna.
Vikonanny dosvidu
In 3 flasks of 200 ml, pour 100 ml of water and add 3 drops of methyl orange indicator. In the pershu flask, drop by drop from the burette of hydrochloric acid and concentration of 0.1 n dot, the water in the flask in the form of chergovy drops does not change the zhovte zabarvlennya on the erysipelas. Indicate the value of vitrified acid to within 0.1 ml. Those same zrobiti with another and third flasks. Razrahuvat average obsyag acid, staining for titration. Calculate the carbonate hardness of water.
Tse water, how to rob beer? Just zanurіt line at the water, as it is necessary to revise it and read it. Reading the results can be done with the help of a program on a mobile phone or with a manual check. Results for chlorine, nitrate, nitrite, hardness, lubricity and pH.
A value of 5 to 5 is ideal. High value is high for brown bacteria and ribi. Anchor test to check 6 different indicators of water quality in less than 60 seconds! Dioxide carbon dioxide gas is different between: - a wide range of carbon dioxide or carbon dioxide - carbon dioxide bicarbonate or sodium carbonate ions - carbon dioxide carbonate ions.
1. Find the molar concentration of equivalents C ek the difference of hydrocarbonates according to the formula (2) (div. example 2).
2. Calculate the carbonate hardness of the water.
W c \u003d C ek ∙ 1000 ml-equiv. / l ion_v Ca 2+ and Mg 2+.
Write down the molecular and ion-molecular reactions that take place during titration.
LABORATORY ROBOT №6
Tsya kіlkіst-vugletseva іvnovaga іstotno to fall in the pH, meadows and temperatures of the water. Razchinennya vapnyak vіdbuvaєtsya until the reach of jealousy. As a rule, do not apply surface water over 10 mg. Deyakі mineral water may be significant, scho exceed 100 mg. Opir leads to the presence of hydroxyl ions, 2-carbonate and bicarbonate, to a lesser extent, to 3-phosphate ions and silicate or to molecular species of weak acids. Closely associated with hardness, its meaning, as a rule, is close, if it is indicated by the presence of carbonates and bicarbonates.
Hardness of water.
Meta robots: by the method of complexonometry to determine the hardness of the water; by the method of neutralization, the carbonate, timchasov and post-hardness are determined.
The hardness of the water is enriched by the presence of calcium and magnesium salts in them. The amount of mg-equion Ca 2+, Mg 2+, which is in 1 liter of water, is called the number of times. 1 mg-equiv (Russian degree of hardness) shows 40.08/2=20.04 mg/l Ca 2+ or 24.3/2=12.15 mg/l Mg 2+.
In natural waters, lubricity, which in bicarbonates, is 10 to 350 mg. Vin is supplemented with the materials of the Mishko chi of the industrial campaign. Alkalimetric titer and the latest alkaline titer reflect the water lubricity. Knowing these meanings can be important for winning, please drive. The stinks lie in the kіlkoh-vugletsevoj r_vnovaga. Zagalna zhorstkіst or hydrotimer name. Tartar at the pipes: the more you drive to avenge the calcium ion, the more you can be an important vapnyakov’s family.
Zmist ions in calcium and magnesium are characterized by a hydrometric titer. Sob zabіgti osadzhennya slag in the pipes, it will be helped by solid water in pom'yakshuvachі. On the back, the hardness turned the building, the water reacted with kindness. The hardness essentially reflects the presence of calcium and magnesium salts. Tse bezporednyo pov'yazane s geological nature of the lands that are intertwined. In this order, the vapnyany or dry soil will give solid water, and the water that melts the crystalline soil, like sand, will give the m'yak water.
Distinguish between hot, carbonate, timchasov and post-yna and non-carbonate hardness.
Zagalnoy zhorstkіstyu called the total concentration of ions Ca 2+, Mg 2+ and salts of calcium, magnesium.
Timchas's hardness part of the hotness is called, which boils water at the atmospheric pressure. Encouraged by the presence of hydrocarbonates of potassium and magnesium, which are laid out when boiling:
Ca (HCO 3) 2 \u003d CaС0 3 + CO 2 + H 2 O
Mg (HCO 3) 2 \u003d Mg (OH) 2 + 2CO 2
Postiynoy zhorstkistyu part of the hardness is called, which is lost after boiling water under atmospheric pressure. It is bound by the presence of calcium and magnesium salts of sulfuric, hydrochloric, nitric, phosphoric and silicic acids, which, when boiled, are saturated with water.
carbonate hardness called part of the hardness, equivalent to the concentration of carbonates and hydrocarbonates of calcium and magnesium.
Non-carbonate hardness part of the hotness is called, there are more differences between the hot and carbonate hardness.
For hardness, water is subdivided into: soft (0-1.5 mg-eq / l of salts), soft (1.5-3), medium hardness (6-10) and hard (more than 10).
According to GOST 1974-82, the amount of water for the state-owned water supply is allowed to become more than 7 mg-eq / l. Salt, which numbs the hardness of the water, is not harsh for living organisms;
The process that reduces the hardness of the water to a reduction, is called help. Help to increase the concentration of calcium and magnesium salts. The main methods of water softening can be divided into three groups: reagent methods: soda-vaping, meadow, barium salts, phosphates; the method of ion exchange with the use of ions; Thermal method.
The severity is determined by the general method - complexometric and neutralization.
Determination of the central and timchas' hardness of the water by complexometricmethod.
The most accurate and widest method for determining the total hardness, bases on the ions of Ca 2+ and Mg 2+ ionized by ions of Ca 2+ and Mg 2+ mіtsnih vnutrіshnokokshnikh spoluk s dvosodium sіllu ethylenediaminetetraoctoic acid Na 2 H 2 Edta or Trilon B:
When titrated with Trilon B, the binding of ions to calcium and magnesium is observed, and they usually go to water, which leads to an increase in the acidity of the water. The accumulation of ions in water is higher than the singing values, it can lead to the destruction of the complex, which, having settled, should be titrated at pH = 8-10. This pH value is reached beyond the buffer ammonium range. The point of equivalence is assigned to an additional metal indicator H2Ind: murexide. acidic chromogen black and others, yakіyut with calcium ions and magnesium less stable complexes, nizh vnutrіshnokompleksnі spoluky Trilon B, which is destroyed in the process of titration. That is why, in terms of equivalence of raspberry-chervone, the zabarvlenny rozchinya goes over to the blue, obumovlenny zabarvlennyam to the anion of the indicator Ind:
H 2 Ind \u003d 2H + + Ind 2- Raspberry-red blue
Ca 2+ + Ind 2- = Ca Ind
Cherry-chervoniy (рН = 8-10)
CaInd + Na 2 H 2 Edta \u003d CaNa 2 Edta + 2H + + Ind 2-
Barless blue
Certificate 1
Hid appointment. At the end of the 250 ml flask, with a pipette, take 100 ml of water, add 5 ml of ammonia buffer sum to the tip of the shoulder blade (sprat of grains) of the indicator. Titrate the stains with a working stain to Trilon B until the cherry-red stain changes to blue. Respect! The overdose of Trilon B has been left unaltered, while the titration of Trilon B has to be added drop by drop. Record the results of the titration.
Rozrahunok. The hardness of the lead should be developed according to the formula (1), the law of equivalences for rozchiniv:
Сн(tr.B) V(tr.B)
Job = 1000, (1)
de Zhob - total hardness of water, mg-equiv/l; Cn(tr.B.) - normality to Trilon B, mol/l; U(tr.B) - total for Trilon B, what is needed for titration, ml; V (H 2 O) - total sample water, ml.
Dosvіd 2. Appointment of temporal and post-mortem hardness.
Hid appointment. At the end of the 250 ml flask with a pipette, add 100 ml of water. Oliver on the skul stands for the ribs of the water at the flask. Close the flask with virva and boil for 1 year. When boiling, the siege is established. At the world of viparovuvannya water in a flask, carefully add distilled water to the mark. After cooling, filter the boiled water through a dry filter into a clean, dry flask, wash the filter 2-3 times with a small amount of water that distills (washing the water with a filtered sample). Away from the appointment of the guardianship (Zhpost) and the rozrahunok to conduct as a witness 1.1.
Rozrahunok timchasova hardness. Timchasov's zhorstkіst vyznajetsya for raznitsa mіzh zagalnoy and postіynoy zhorstkіst.
Zhvr = Zhob. - Zpost.
Determination of carbonate hardness by the method of neutralization.
The method is based on the reaction of neutralization - interchange between acid and meadow. The point of equivalence is determined by vicarious acid-base indicators.
Certificate 3. Designation of carbonate hardness
The method of bases on the binding of ions of HCO 3 - and CO 3 2 - acid in the presence of methyl orange. When titrated, reactions occur:
3 2- + HCO 3 - ; HCO 3 - + H + = CO 2 + H 2 O
Hid appointment. At the end of the 250 ml flask with a pipette add 100 ml of water, add 2 drops of methyl orange. Titrate the sample with 0.1 n working solution of hydrochloric acid until the color changes from yellow to orange. Record the results of the titration.
Rozrahunok. The carbonate hardness should be developed according to the formula (2), the vicarious law of equivalents for rozchinіv:
CH(HCl) V(HC1)
Pk = 1000 , (2)
de Zhk - carbonate hardness, mg-equiv/l; Cn(HC1) – normality of the working range of hydrochloric acid, ml; V(HC1) - total amount of hydrochloric acid, which is needed for titration, ml; V (H 2 O) - total sample water, ml.
Visnovok:
The food is the same.
1. What is called hardness? Indicate one in the world of hardness. Pererahuyte class of hardness, see the hardness.
2. How do they signify the hardness of the water? Describe the methods, write down the equal reactions.
3. Describe the ways to mitigate the drive. Write down the equal reactions.
4. What are ion-exchange resins: cations, anions? Bring examples. Give me an understanding of that rozrahunok exchange capacity. What is the regeneration of ions. What advantages can ion exchange method have over reagent methods?
5. What is amplification? Galuzy zastosuvannya. Perevaga that is not enough for the method.
7. How much 3% HCI is needed to convert carbonate hardness (8 mg-eq / l) 4 m 3 of water, which vicorizes in the heat exchanger, to transfer to non-carbonate?
8. For sodium phosphate, it is necessary to add up to 500 liters of water to reduce carbonate hardness, which is about 5 mt-eq / l.
9. Water analysis found: non-carbonate hardness is 3.0 mg-eq/l; carbonate hardness - 4.5 mg-eq / l; magnesium hardness 2.4 mg-eq/l; free CO2 - 8 mg-eq/l. Razrahuvati dose of vapne (in pererakhunku on CaO) and the dose of anhydrous soda.
10. Calculate the amount of calcium ion, which is used to ignite I l cation, so that the working capacity is 730 g-eq / m 3.




